Capillary Water
When You Talking About Important Topic in Soil mechanics and Also in other Engineering Subject Capillary Water Surface tension is one of the most important Topic.
This is lesson 1 in which we talk about only Capillary water basic and surface tension.
So the Question 1 is What is Capillary Water?
Before Discussing Capillary water it Very important to see this Graph of the type of soil water.
Free Water In soil –
these water flows from one point to other were different of total head Present.
mainly reason of this is due to an influence of the gravity. Now we know the particular space in soil is very low so this water is a laminar Flow type. ( maybe non-laminar in Case of coarse soil)
Free Water Give the Permeability Effect in soil
Held Water-
it does not move under the influence of the gravity, basically held water is retained in the Pores of the soil.
Structure Water – This water is Part of the crystal structure of the mineral of the soil. it chemically combined with soil structure so consider a part of the soil structure. a removal to this water Required Very high temperature and soil mineral breaking.
Adsorbed Water – firstly not read it as Absorbed, it’s a different type of water which around the soil particles surface and make a layer over particle. this layer act like glue and more particle with adsorbed water attracts and form a soil mass.
Adsorbed water present depend on clay minerals. and it gives the plastic characteristic to soil mass.
Capillary water – these water held in soil due to a capillary force (Inter particular attraction of water molecules to the solid surface )
So the Question 2 is
What is Surface Tension
Surface Tension – It is Basically Force per unit length of line Drawn on the surface. to understand Surface tension Properly see the diagram and know the practical definition.
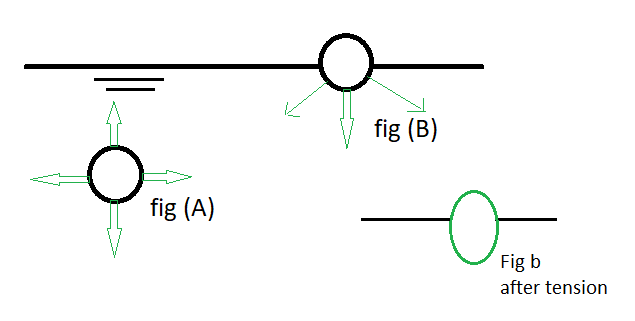 |
| surface tension in soil |
In fig (A) force due to the Molecular attraction act all round and Molecular in equilibrium.
In fig (B) Molecule is surrounding by to Pull,
firstly air pulls upward and on the bottom water molecule pull downward.
as we know water pull is higher than air so molecule shape is like eggs.
Now the practical definition of surface tension is – tension produced due to water and air pull to the molecule. these tensions give force production on the surface per unit length.
Value of the surface tension at the normal temperature is 0.073N/m.